Interpretation of osmotic consolidation phenomena through local measurements of displacement and cation concentration
Swelling clays such as bentonite are layered minerals composed of negatively charged clay crystals. Cations in the pore water are attracted to the negatively charged mineral crystals. The difference in ionic concentration between the crystals (inside the interlayer) and the pore water generates osmotic pressure, which causes water to move between the crystal layers and the pore water. When the pore water concentration is (relatively) high, water drains from between the crystal layers, and when the concentration is low, water is absorbed by the crystal layers. Although this osmotic consolidation phenomenon has been known for a long time, it has only been analyzed macroscopically in terms of the amount of settlement in relation to ionic concentration as an elemental test.
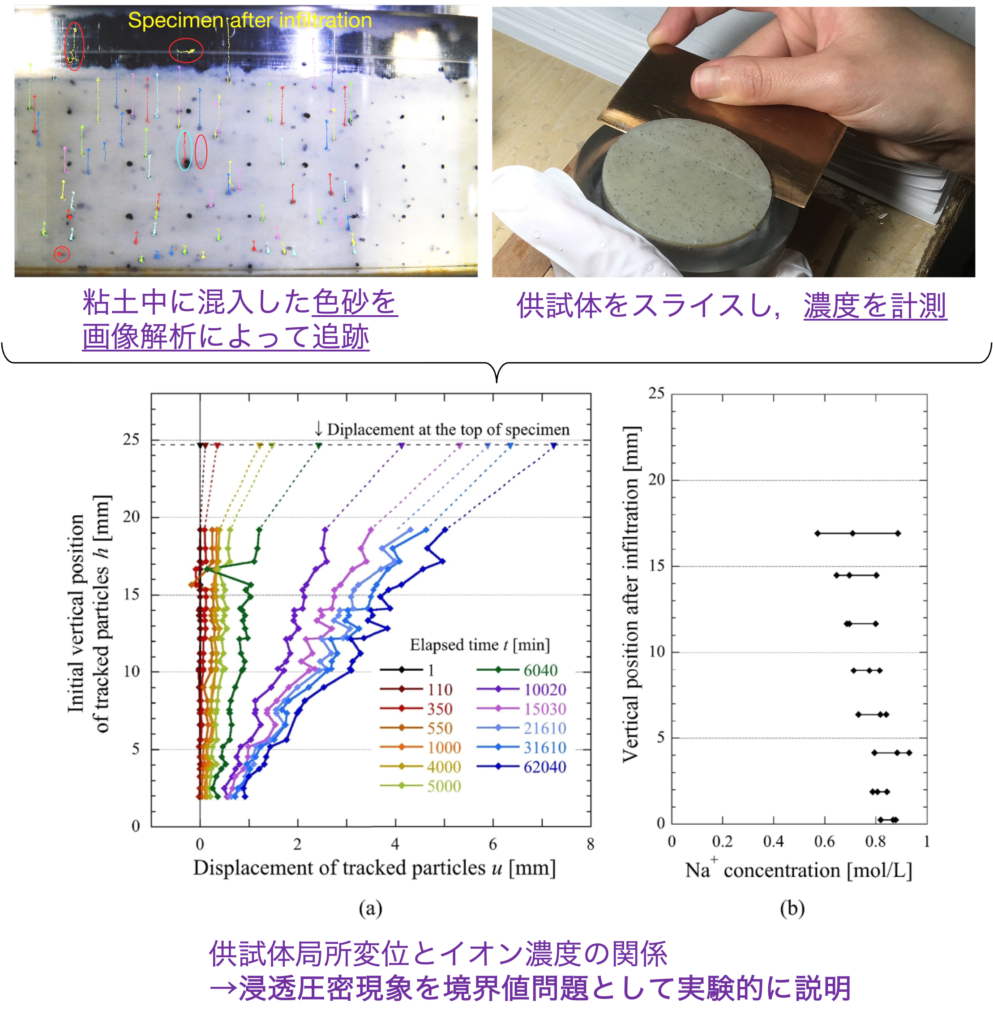
In this study, in order to consider this osmotic consolidation phenomenon as a boundary value problem, (1) local displacement was extracted by observing the movement of colored sand mixed into the specimen using an acrylic consolidation vessel by image analysis, and (2) the local ion concentration was measured by slicing the specimen after osmotic consolidation. This enabled a spatial understanding of temporal changes in ion concentration and local displacement. It was also shown that not only ion concentration but also pore pressure is involved in the progress of seepage consolidation, and the phenomenon was explained as a coupled hydraulic-mechanical-chemical-chemical problem.
Hiraga, M., Kyokawa, H. and Koseki, J.: Experimental investigation of local chemo-mechanical behaviour and temporal development of osmotic consolidation in expansive clay, Canadian Geotechnical Journal. (Ahead of print)